Can ionization induce an enhancement of hydrogen storage in Ti2–C2H4 complexes?
Abstract
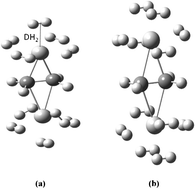
We predicted here gravimetric hydrogen storage capacity of Ti2–C2H4 and Ti2–C2H4+ complexes using density functional theory (DFT) method. The number of adsorbed H2 on Ti2–C2H4 is limited to ten. It has been seen that controlling the charge-state of this complex enhances its gravimetric hydrogen uptake and the metal bond strength. The resulting cationized complex then adsorbs two additional H2 molecules than the neutral complex. In addition, we elucidated the effect of exchange and correlation functionals in DFT on H2

 Please wait while we load your content...
Please wait while we load your content...