Formal total synthesis of (−)-exiguolide†
Abstract
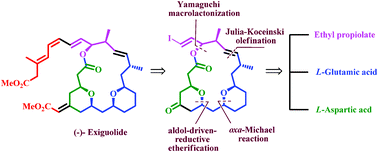
The formal total synthesis of (−)-exiguolide through the chiral-pool approach is described. The key reactions involved for formation of the macrocyclic core from two subunits are Julia–Kocienski olefination and Yamaguchi macrolactonization. The major methylene bis-tetrahydropyran fragment was achieved in a convergent manner from L-glutamic acid and L-aspartic acid involving the oxa-Michael reaction and an aldol-driven reductive etherification as key steps for the formation of a tetrahydropyran ring. The other sulfone subunit was prepared via an Evans aldol reaction.

 Please wait while we load your content...
Please wait while we load your content...