Molten salt synthesis and energy storage studies on CuCo2O4 and CuO·Co3O4
Abstract
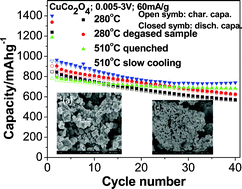
CuCo2O4 and CuO·Co3O4 compounds were prepared by a one-pot simple molten salt method (MSM) at 280 °C to 750 °C. Changes in morphology, crystal structure and electrochemical properties of CuCo2O4 as a function of preparation temperatures were investigated using

 Please wait while we load your content...
Please wait while we load your content...