Electrochemical performance of NASICON type carbon coated LiTi2(PO4)3 with a spinel LiMn2O4 cathode†
Abstract
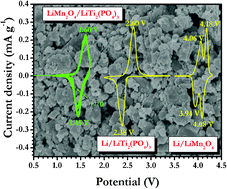
NASICON type LiTi2(PO4)3 particles are synthesized by a modified pechini type polymerizable complex method at 1000 °C in an air atmosphere. The synthesized LiTi2(PO4)3 particles are ball milled and subsequently carbon coated from the carbonization of

 Please wait while we load your content...
Please wait while we load your content...