Novel catalyst layer synthesized by an in situ sol–gel process with tetraethoxysilane in a Nafion ionomer solution with Pt/C for PEFCs: the effect of self-assembled Nafion–SiO2 on Pt ORR activity and an increased water content in the polymer membranes†
Abstract
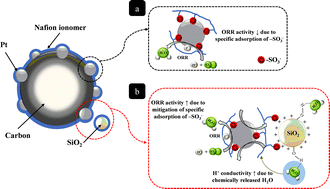
A highly optimized catalyst layer, under low humidity conditions, was developed through an in situ sol–gel process with tetraethoxysilane (TEOS) in a Nafion ionomer solution with Pt/C in order to prepare a polymer electrolyte fuel cell (PEFC). This method is quick and does not require the use of additional solvents to accomplish the sol–gel reaction of TEOS at high temperatures compared to other ex situ sol–gel methods. Amorphous SiO2 nanoparticles of <5 nm were distributed uniformly in the vicinity of the graphitic carbons and the Pt electrocatalysts without significant particle aggregation. In the in situ sol–gel reaction, due to their very high acidity, –SO3H groups attached to the Nafion polymer conveniently served as catalyst to facilitate the hydrolysis reaction. Hydrolyzed alkoxysilanes migrated to the negatively charged sulfonate groups (–SO3−) due to a rapid increase in the ζ potential of the SiO2 nanoparticles at pH < 2, which resulted in a core–shell structure in the catalyst ink during the drying of the membrane–electrode assemblies (MEAs). A core–shell structure that consisted of positively charged ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiOH2+ groups and negatively charged –SO3− in the Nafion ionomer was evaluated for its enhancement of the water content in the polymer membranes, as well as its effect on the specific activity of Pt. When an MEA has SiO2 nanoparticles only within the cathode catalyst layer under low humidity, the chemically-adsorbed water molecules on the silanol groups are released into the cathode catalyst layer, increasing the water content at the cathode catalyst layer, which increases the water transport from the cathode to the anode (i.e., back diffusion). The enhanced back diffusion mitigated the dehydration of the membrane under low humidity conditions. Furthermore, it is interesting to note that the core–shell structure between positively-charged
SiOH2+ groups and negatively charged –SO3− in the Nafion ionomer was evaluated for its enhancement of the water content in the polymer membranes, as well as its effect on the specific activity of Pt. When an MEA has SiO2 nanoparticles only within the cathode catalyst layer under low humidity, the chemically-adsorbed water molecules on the silanol groups are released into the cathode catalyst layer, increasing the water content at the cathode catalyst layer, which increases the water transport from the cathode to the anode (i.e., back diffusion). The enhanced back diffusion mitigated the dehydration of the membrane under low humidity conditions. Furthermore, it is interesting to note that the core–shell structure between positively-charged ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiOH2+ groups and negatively-charged –SO3− groups in the Nafion ionomer may reduce the specific adsorption of –SO3− groups on the surface of Pt, which enhances the kinetics of the oxygen reduction reaction (ORR) in the catalyst layer of the cathode.
SiOH2+ groups and negatively-charged –SO3− groups in the Nafion ionomer may reduce the specific adsorption of –SO3− groups on the surface of Pt, which enhances the kinetics of the oxygen reduction reaction (ORR) in the catalyst layer of the cathode.

 Please wait while we load your content...
Please wait while we load your content...