Electrochemical kinetics of the 0.5Li2MnO3·0.5LiMn0.42Ni0.42Co0.16O2 ‘composite’ layered cathode material for lithium-ion batteries
Abstract
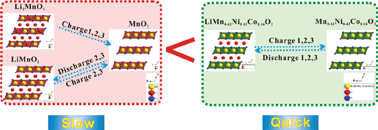
The ‘composite’ layered material of 0.5Li2MnO3·0.5LiMn0.42Ni0.42Co0.16O2 has been successfully prepared by the solid state reaction method, and was characterized by

 Please wait while we load your content...
Please wait while we load your content...