Combined IR absorption and modeling study of nanoporous zeolite imidazolate frameworks (ZIFs) filled with hydrogen
Abstract
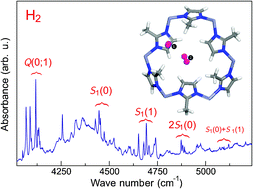
A combined IR absorption and first principles modelling study of zeolite imidazolate frameworks (ZIFs) filled with hydrogen is presented. It is shown that hydrogen physisorbed in a ZIF results in a number of absorption lines at around 4131, 4121, 4480, 4700, 4880, 5100, and 5280 cm−1, which are assigned to the Q(0), Q(1), S1(0), S1(1), 2S1(0), S1(0) + S1(1), and 2S1(1) ro-vibrational transitions of physisorbed H2, respectively. The latter three modes represent simultaneous excitation of molecular pairs, which implies that hydrogen physisorbed in ZIF occurs at a density close to that of the liquid and/or solid state. The

 Please wait while we load your content...
Please wait while we load your content...