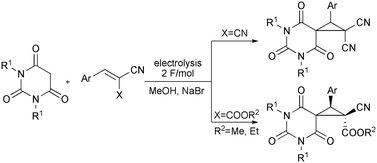
Electrocatalysis in MIRC reaction strategy: facile stereoselective approach to medicinally relevant spirocyclopropylbarbiturates from barbituric acids and activated olefins†
Abstract
The combined electrolysis of barbituric acids and benzylidenemalononitriles or benzylidenecyanoacetates in

 Please wait while we load your content...
Please wait while we load your content...