Synthetic 5′-phosphorylated oligodeoxynucleotide purification through catching full-length sequences by polymerization†
Abstract
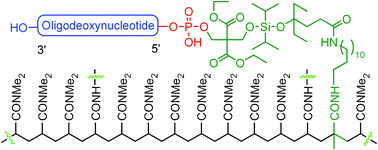
The readily scalable catching by polymerization purification technology has been further advanced to purify 5′-phosphorylated synthetic oligodeoxynucleotides (ODNs). The new technology utilizes a phosphoramidite that contains a fluoride-cleavable diisopropylsilyl acetal linker and a polymerizable methacrylamide group, and is capable of phosphorylation of ODN. For purification, the phosphoramidite was coupled to the 5′-end of full-length ODN on a synthesizer. Because failure sequences were capped in each synthetic cycle, only the full-length sequences were phosphinylated and acrylated. After cleavage and deprotection, the crude ODN was subjected to polymerization under typical acrylamide gel formation conditions. The full-length ODN was incorporated into polymer. The failure sequences and other impurities were simply removed by washing with water. Pure full-length ODN that contained a 5′-phosphate group was cleaved from the polymer with HF–pyridine. Reversed-phase (RP) HPLC showed that the ODN was pure, and the recovery yield was higher than that of typical preparative HPLC purification.

 Please wait while we load your content...
Please wait while we load your content...