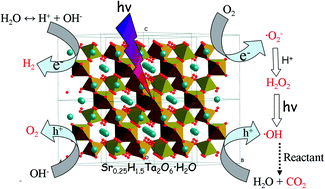
A new photocatalyst Sr0.25H1.5Ta2O6·H2O (HST) with high surface area was successfully prepared by a facile and mild hydrothermal reaction using Ta2O5·nH2O as a precursor. TEM images revealed that their different morphologies, from nanoplate to nanopolyhedron, were formed under different pH values of the reactive solutions. The formation mechanism of HST was also studied and proposed. Growth of HST nanocrystallites followed a reaction-crystallization model. By analyzing the results of XRD, DRS, XPS, electrochemistry and theoretical calculation, the crystal and electronic structural characterizations of HST was established. Compared with isostructural Sr0.4H1.2Nb2O6·H2O (HSN), the Ta 5d orbitals were the main contribution to the bottom of the conduction band of HST, inducing the energy level more negative while the top of valence band, which was dominated by O 2p states, remained almost unchanged. Due to the more suitable electronic band structure and various morphologies, the photocatalyst showed superior photocatalytic activities for water splitting to generate H2 and for degrading benzene as compared with HSN. The rate of H2 evolution and the conversion ratio of benzene were 81 times and 4 times higher than that of TiO2 (Degussa P25), respectively. The proposed mechanisms for photocatalytic reactions based on the experimental results were discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...