Development of LPS antagonistic therapeutics: synthesis and evaluation of glucopyranoside-spacer-amino acid motifs†
Abstract
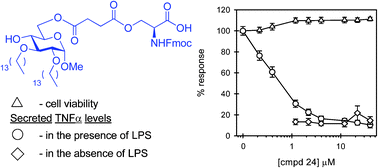
Sepsis is a serious medical condition characterized by bacterial infection and a subsequent massive systemic inflammatory response. The release of proinflammatory products and mediators from responding innate immune cells, such as mononuclear phagocytes, directly contributes to the pathogenesis of sepsis. The primary bacterial trigger of inflammation is

 Please wait while we load your content...
Please wait while we load your content...