Stereoselective synthesis of 2-trifluoromethylated and 2-difluoromethylated dihydrofurans via organocatalytic cascade Michael/alkylation reaction†
Abstract
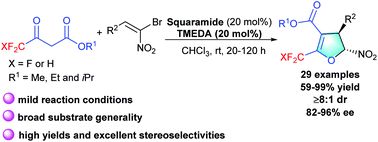
A highly stereoselective cascade Michael/alkylation process has been established to construct 2-trifluoromethylated and 2-difluoromethylated dihydrofurans from readily available starting materials. In the presence of a bifunctional squaramide, the annulation approach proceeded smoothly to afford the desired fluorinated dihydrofurans in 59–99% isolated yields with 82–96% ee and 8 : 1–>20 : 1 dr. This catalytic strategy was well compatible with various structurally distinct β,β-bromonitrostyrenes and fluorinated acetoacetates. Profound synthetic manipulation could be performed on the resulting products.


 Please wait while we load your content...
Please wait while we load your content...