Intramolecular Alder-ene cycloisomerization of cyclopropenes with alkenes to access spirocycles†
Abstract
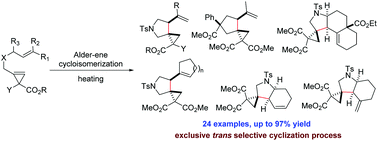
Cyclopropenes carrying an alkene moiety undergo a stereoselective Alder-ene cycloisomerization under thermal conditions. Diverse functionalized (hetero)spiro[2.4]heptanes are obtained with excellent trans diastereoselectivity. According to computational studies, this thermal process takes place via transition state TS(E-exo) or TS(Z-endo) if the former is not available.


 Please wait while we load your content...
Please wait while we load your content...