Highly regio- and stereoselective synthesis of bis-sulfanyl substituted conjugated dienes by copper–palladium cooperative catalysis†
Abstract
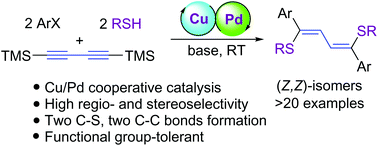
An efficient one-pot method for the synthesis of symmetric (Z,Z)-isomers of 1,4-bis(sulfanyl)-1,4-diaryl-1,3-butadienes from the easily available aryl halides, 1,4-bis(trimethylsilyl)butadiyne and thiols by the cooperative catalysis of Cu(Xantphos)I and Pd(OAc)2 and a base has been developed. Preliminary mechanistic studies revealed that the reaction probably follows the double-Sonogashira coupling/double hydrothiolation pathway, in which two C–C bonds and two C–S bonds were formed sequentially. This protocol features a good substrate scope with a broad range of functional group tolerances, simplicity of operation, and high regio- and stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...