Copper-catalyzed, N-directed remote C(sp3)–H azidation and thiocyanation†
Abstract
An efficient and general method for the catalytic installation of azido and thiocyanato groups via remote C(sp3)–H activation is described. The copper-catalyzed reaction proceeds through a cascade of single-electron transfer, favorable 1,5-hydrogen atom transfer, and C–N/C–S cross-coupling, thus providing the distal azido and thiocyanato alkylamines. This reaction could be applicable to primary, secondary, tertiary, and benzylic C(sp3)–H functionalization, as well as demonstrating excellent site-selectivity and functional-group compatibility.
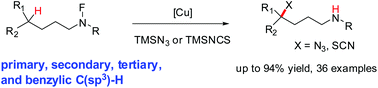


 Please wait while we load your content...
Please wait while we load your content...