Site-selective and diastereoselective functionalization of α-amino acid and peptide derivatives via palladium-catalyzed sp3 C–H activation
Abstract
α-Amino acid and peptide derivatives are important in synthetic organic chemistry and medicinal chemistry, which attract many chemists to develop new methods for their synthesis. Palladium-catalyzed sp3 C–H activation/functionalization has been developed greatly for the modification of α-amino acids and peptides. This review introduces palladium-catalyzed sp3 C–H activation/functionalization of amino acids and peptides. The content includes intermolecular functionalization (arylation, alkylation, alkenylation, alkynylation, acetoxylation, fluorination, silylation, borylation, sulfonylation, chalcogenation and carbonylative cyclization) and intramolecular functionalization (intramolecular C–N bond-forming cyclization and peptide macrocyclization).
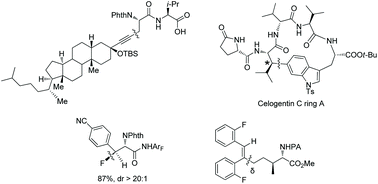
- This article is part of the themed collection: 2021 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...