The dual alkylation of the C(sp3)–H bond of cyclic α-methyl-N-sulfonyl imines via the sequential condensation/hydride transfer/cyclization process†
Abstract
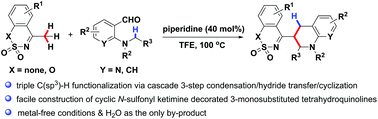
The dual alkylation of the C(sp3)–H bond of cyclic α-methyl-N-sulfonyl imines has been achieved through the piperidine-promoted cascade condensation/[1,5]-hydride transfer/cyclization sequence from cyclic α-methyl-N-sulfonyl imines and o-aminobenzaldehydes in trifluoroethanol, providing structurally diverse 3-cyclic N-sulfonyl imine substituted tetrahydroquinolines in good yields with excellent diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...