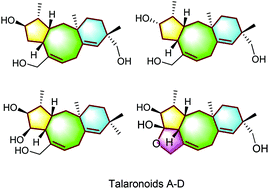
Talaronoids A–D: four fusicoccane diterpenoids with an unprecedented tricyclic 5/8/6 ring system from the fungus Talaromyces stipitatus†
Abstract
Talaronoids A–D (1–4), four diterpenoids with an unexpected tricyclic 5/8/6 carbon skeleton isolated from Talaromyces stipitatus, represent a new class of fusicoccane diterpenoids and the first examples of natural products with a benzo[a]cyclopenta[d]cyclooctane skeleton. Their structures were established by a combination of extensive spectroscopic analyses and electronic circular dichroism spectral calculations. Plausible biosynthetic pathways of 1–4 are proposed starting from geranylgeranyl diphosphate with a Wagner–Meerwein rearrangement as the key step. Compounds 1–4 were evaluated for butyrylcholinesterase inhibitory activity in vitro, and compounds 1–4 showed moderate activity.


 Please wait while we load your content...
Please wait while we load your content...