Synthesis of pertrifluoromethyl pyridazine derivatives via a tandem reaction of aryldiazonium salts with hexafluoroacetylacetone†
Abstract
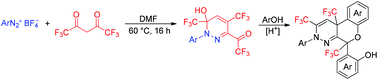
A new strategy for the construction of pertrifluoromethyl pyridazine derivatives is presented. The tandem reaction starts from the condensation of arenediazonium salts with hexafluoroacetylacetone leading to the formation of pertrifluoromethyl pyridazinols, which readily undergo electrophilic substitution reactions with phenols to afford benzo[5,6]chromeno[3,4-c]pyridazine products in the presence of concd H2SO4.


 Please wait while we load your content...
Please wait while we load your content...