Nickel-catalyzed hydroalkenylation of styrene with phenylpropanal: theoretical studies on the mechanism, regioselectivity, and role of phenylboronic acid†
Abstract
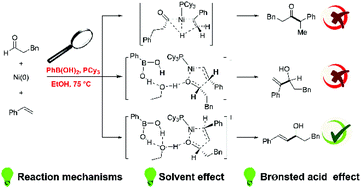
The reaction mechanism of nickel-catalyzed hydroalkenylation of aldehydes with styrene enabled by a Brønsted acid has been theoretically investigated by employing density functional theory (DFT) calculations. Based on our mechanistic study, this reaction includes three main steps: oxidative cyclization, ring opening of an oxanickellacycle, and a β-hydride elimination mechanism via an agostic interaction. The regioselectivity for forming a linear or branched allylic alcohol is controlled by the oxidative cyclization step. The origin of the regioselectivity is explored by analyzing the electronic and steric effects. The role of phenylboronic acid and ethanol solvent in enhancing the reactivity and preventing the formation of a branched ketone product could be attributed to the hydrogen bond interaction in the oxidative cyclization step. The effects of different solvents, Brønsted acids, and ligands on the reactivity are also clarified through our calculations.


 Please wait while we load your content...
Please wait while we load your content...