Rhodium(iii)-catalyzed synthesis of 3-trifluoromethylindanones from N-methoxybenzamides via C–H activation and Claisen/retro-Claisen reaction†
Abstract
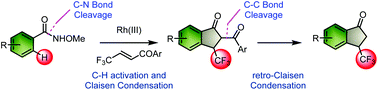
A rhodium(III)-catalyzed reaction of N-methoxybenzamides as a directing group with β-trifluoromethyl-α,β-unsaturated ketones is reported. The reaction involved sp2 C–H activation, followed by the Claisen condensation involving a C–N bond cleavage to form 2-acyl-3-trifluoromethylindanone products. Furthermore, C2-unsubstituted 3-trifluoromethylindanone formation occurs through the trifluoroethanol-mediated retro-Claisen condensation reaction. This protocol is an efficient, straightforward route for the synthesis of 3-trifluoromethylindanone derivatives.


 Please wait while we load your content...
Please wait while we load your content...