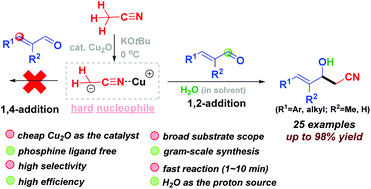
Cu2O-catalyzed selective 1,2-addition of acetonitrile to α,β-unsaturated aldehydes†
Abstract
A cyanomethylation of α,β-unsaturated aldehydes with acetonitrile as a nucleophile is disclosed. The reaction is promoted with Cu2O as an inexpensive catalyst. Mild conditions are used, and the reaction completes within a few minutes. Through our simple and scalable operation, a large set of δ,γ-unsaturated-β-hydroxyl nitriles are prepared in good to high yields.


 Please wait while we load your content...
Please wait while we load your content...