Straightforward access to densely substituted chiral succinimides through enantioselective organocatalyzed Michael addition of α-alkyl-cyclic ketones to maleimides†
Abstract
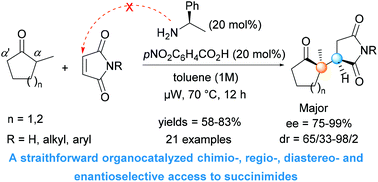
A simple organocatalytic system provides efficient access to a series of densely substituted chiral succinimides bearing a quaternary–tertiary carbon stereocenter sequence in good yields, with high diastereo- and enantioselectivities through enantioselective conjugate addition of unreactive α-alkyl cyclic ketones to maleimides under microwave-assisted conditions.


 Please wait while we load your content...
Please wait while we load your content...