Ru-Catalyzed cascade reaction of α,ω-alkynoic acids and arylethylamines towards the synthesis of aryl-fused heterocycles†
Abstract
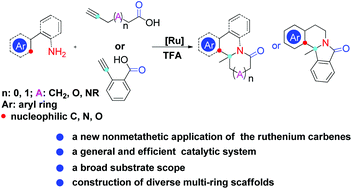
A simple, efficient, and mild method for the preparation of aryl-fused heterocycles has been developed using α,ω-alkynoic acids and arylethylamines. In the present method, one of Grubbs’ ruthenium carbenes is able to catalyze the formation of exocyclic enol lactones from α,ω-alkynoic acids, which subsequently undergo aminolysis and N-acyl iminium ion formation/cyclization with arylethylamines in the presence of TFA, affording one kind of “drug-privileged” scaffold. Demonstrated with high functional group tolerance, this new cascade reaction endows the Grubbs’ ruthenium carbene with a new synthetic utility beyond routine olefin metathesis.


 Please wait while we load your content...
Please wait while we load your content...