Rhodium-catalyzed homodimerization–cyclization reaction of two vinyl isocyanides: a general route to 2-(isoquinolin-1-yl)oxazoles†
Abstract
A novel rhodium-catalyzed homodimerization–cyclization reaction of two vinyl isocyanides has been developed for the first time. In this reaction, the highly reactive 1,4-diazabutatriene intermediates generated by the homodimerization of two vinyl isocyanides could undergo a novel type of intramolecular tandem cyclization and provides a new and highly efficient method for the construction of 2-(isoquinolin-1-yl)oxazoles by formation of three new bonds and two rings from readily available acyclic starting materials in a single step.
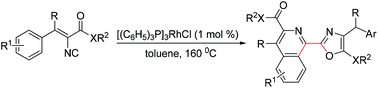


 Please wait while we load your content...
Please wait while we load your content...