An enantioselective aza-Henry reaction of trifluoromethyl ketimines catalyzed by phase-transfer catalysts†
Abstract
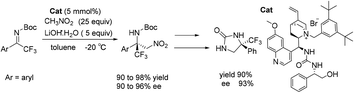
An efficient enantioselective aza-Henry reaction of acyclic trifluoromethyl-substituted ketimines has been performed successfully by using a bifunctional chiral ammonium catalyst bearing multiple H-bonding donors, which was derived from quinine. The corresponding products were obtained in excellent yields (up to 98% yield) with high enantioselectivities (up to 96% ee), and they could be easily converted to diamines and imidazolidine-2-ones with chiral tetrasubstituted carbon centers in excellent yields with high enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...