Asymmetric diastereodivergent Michael addition of 2-chloromalonate esters to conjugated imines enabled by catalyst metal change†
Abstract
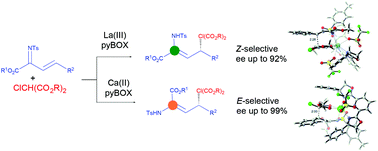
Despite recent progress in asymmetric diastereodivergent reactions leading to products bearing two stereogenic centers, little research has been devoted to processes were a stereogenic center and a double bond are formed. Here, we report the asymmetric diastereodivergent Michael addition of 2-chloromalonate esters to N-tosyl β,γ-unsaturated α-ketimino esters to give chiral α,β-dehydro-α-aminoesters bearing either a Z or E enamine moiety, using pyBOX-metal complexes. Diastereodivergency is achieved by simply changing the metal ion from a trivalent La(III) to a divalent Ca(II) ion, providing the Z or E enamines. Computational studies reveal the crucial role of London interactions between the aromatic residues on the imine and the pyBOX ligand, which are conditioned by the different coordination modes of La(III) and Ca(II), in the change of selectivity depending on the used metal.


 Please wait while we load your content...
Please wait while we load your content...