The synthesis and properties of a new class of π-expanded diketopyrrolopyrrole analogs and conjugated polymers †
Abstract
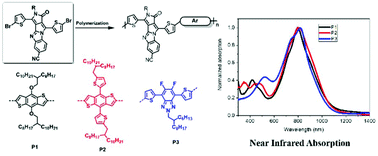
A novel 6-5-5-5 ring containing mono-ketopyrrole in its fully conjugated state, resembling a π-expanded diketopyrrolopyrrole analog, has been designed and synthesized. The new type of molecule shows intense visible range absorption and a reversible reduction potential. Further, this class of molecule can be incorporated into a family of alternative conjugated polymers. These new polymers possess planar backbones due to the fused π-expanded diketopyrrolopyrrole analog building block and smaller steric effects between the thiophene of azaanthracene and the flanking thiophene rings of the comonomer units. These new very low band gap polymers exhibit NIR absorption spectra that are extended to 1200 nm due to the extended effective conjugation length and increased molecular orbital overlap between aromatic monomer units. Our results demonstrate that these π-expanded diketopyrrolopyrrole analogs could be a useful building block for semiconducting polymers.


 Please wait while we load your content...
Please wait while we load your content...