Synthesis of rigidified shikimic acid derivatives by ring-closing metathesis to imprint inhibitor efficacy against shikimate kinase enzyme†
Abstract
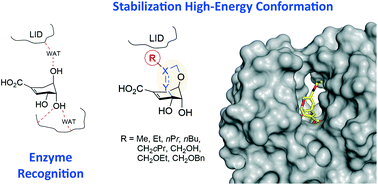
Diverse rigidified shikimic acids derivatives, which are stable mimetics of the high-energy conformation of shikimic acid, have been synthesized to enhance inhibitor efficacy against shikimate kinase enzyme (SK), an attractive target for antibiotic drug discovery. The synthesis of the reported conformationally restricted shikimic acid derivatives was carried out by ring-closing metathesis of allyloxy vinyl derivatives as the key step. The rigidification of the ligand conformation was used to maximize the effectiveness of the substituents introduced in the ether carbon bridge of the scaffold by pre-orienting their interaction with key residues and enzyme domains that are essential for catalysis and enzyme motion. Molecular Dynamics simulation studies on the enzyme/ligand complexes revealed marked differences in the positioning of the ligand substituent in the active site of the two enzymes studied (SK from Mycobacterium tuberculosis and Helicobacter pylori) and this explains their greater efficacy against one of the enzymes. This enhancement is due to the distinct induced-fit motion of the two homologous enzymes. A 20-fold improvement against the H. pylori enzyme was achieved by the introduction of a CH2OEt group in the rigid ether bridge of the reported shikimic acid analogs.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...