Copper-catalysed C(sp3)–N coupling initiated by selective C–C bond cleavage of cyclobutanone oxime esters†
Abstract
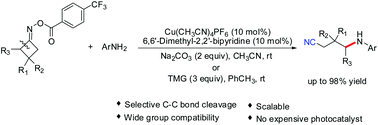
Here we report an efficient copper-catalysed selective C–C bond cleavage/amination of cyclobutanone oxime esters. This reaction protocol is operationally simple and conducted at ambient temperature, allowing access to a wide range of functionalized 4-(arylamino)butanenitriles in moderate to excellent yields. This transformation shows high chemo-selectivity and wide functional-group compatibility and can be easily scaled up to the gram level with a useful yield. A mechanism involving copper-catalysed capture of alkyl radical intermediates by amine nucleophiles is proposed.


 Please wait while we load your content...
Please wait while we load your content...