Visible light enabled γ-trifluoromethylation of Baylis–Hillman acetates: stereoselective synthesis of trisubstituted alkenes†
Abstract
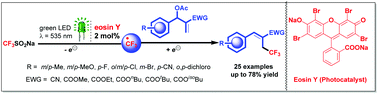
A distinct visible light induced γ-trifluoromethylation of Baylis–Hillman acetates has been accomplished using eosin Y as a photoredox catalyst and bench stable Langlois’ reagent (CF3SO2Na) as a CF3 source under metal-free conditions at room temperature to afford various trisubstituted alkenes. The product stereochemistry is predominantly trans (E), and involves trifluoromethylation followed by de-acetoxylation.


 Please wait while we load your content...
Please wait while we load your content...