A general synthesis of unnatural α-amino acids by iron-catalysed olefin–olefin coupling via generated radicals†
Abstract
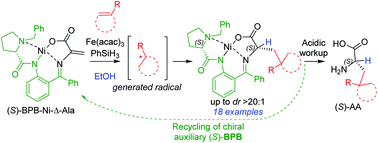
Herein, we report an efficient and practical protocol for the asymmetric synthesis of unnatural α-amino acids (AAs) with γ-tertiary and quaternary carbon centres via selective intermolecular iron-catalysed olefin–olefin coupling of a chiral Ni(II) complex of dehydroalanine Schiff base 2 ((S)-BPB-Ni-Δ-Ala) with different olefins. In all successful reactions, diastereomeric nickel complexes were typically obtained in a ratio of up to dr > 20 : 1. The desired products were isolated in 42–93% yields (18 examples). Exemplarily, the three Ni(II) complexes obtained were decomposed with aqueous HCl and the target unnatural α-amino acids were isolated with excellent enantioselectivities and yields of up to 83%. The chiral auxiliary (BPB = 2-(N-benzylprolyl)aminobenzophenone) was readily recycled and reused for the synthesis of the starting chiral Ni(II) complex 2. The developed protocol provided a facile access to the analogues of glutamic acid, leucine and phosphinothricin.


 Please wait while we load your content...
Please wait while we load your content...