An efficient synthesis of fluoro-neplanocin A analogs using electrophilic fluorination and palladium-catalyzed dehydrosilylation†
Abstract
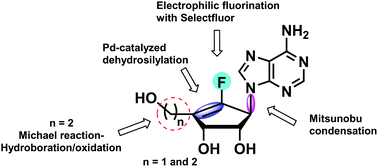
Neplanocin A analogs have attracted attention in the fields of biological and medicinal chemistry due to their potent and broad-spectrum antiviral and antitumor activities. To further explore their potential as therapeutic agents, an alternative and efficient synthesis of fluoro-neplanocin A analogs has been developed by employing stereoselective electrophilic fluorination and palladium-catalyzed dehydrosilylation as key steps. With respect to previous syntheses, the present synthetic methodology provides easy access to key intermediates that could contribute to expanding further the structure–activity relationship studies of neplanocin A analogs.


 Please wait while we load your content...
Please wait while we load your content...