Additive-free regio- and diastereoselective construction of fully-substituted isoxazolidines employing diazo compounds†
Abstract
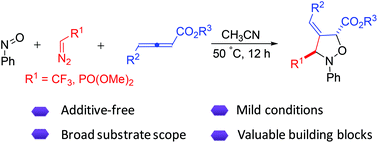
An efficient additive-free three-component reaction of trifluorodiazoethane, nitrosobenzene, and allenic esters has been uncovered. This protocol provides easy access to a variety of trifluoromethylated isoxazolidines with good functional group tolerance. Moreover, this methodology was found to be extendable to α-diazomethylphosphonate providing phosphonylated isoxazolidines. The utility of these products has been demonstrated by the expedient synthesis of trifluoromethylated lactam.


 Please wait while we load your content...
Please wait while we load your content...