Insights into highly selective ring expansion of oxaziridines under Lewis base catalysis: a DFT study†
Abstract
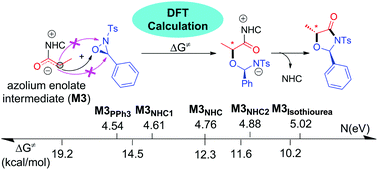
The competing mechanisms of the highly selective ring expansion reaction of oxaziridines catalyzed by N-heterocyclic carbenes (NHCs) have been theoretically studied by density functional theory (DFT). The calculated results indicate that the overall catalytic cycle includes two stages, i.e., the formation of an enolate intermediate and the ring expansion of oxaziridines. The C–O bond formation step in stage 2 was found to be both the stereoselectivity-determining and rate-determining step. The non-covalent interaction (NCI) analysis indicates that the R-configurational pathway has more hydrogen bonds so that the corresponding product is energetically favorable. The global reactivity index (GRI) analysis reveals that the enolate intermediate is the nucleophilic reagent. Furthermore, the calculated results indicate that the efficiency of different Lewis base organocatalysts would be predicted by calculating the nucleophilicity index N values of the corresponding enolate intermediates.


 Please wait while we load your content...
Please wait while we load your content...