Enantioselective synthesis of trifluoromethylated dihydroquinoxalinones via palladium-catalyzed hydrogenation†
Abstract
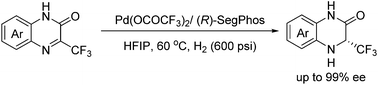
A highly enantioselective palladium-catalyzed asymmetric hydrogenation of 3-(trifluoromethyl)quinoxalinones has been successfully developed, providing a general and facile access to chiral 3-(trifluoromethyl)-3,4-dihydroquinoxalinones with up to 99% ee. In addition, the 3-(trifluoromethyl)-3,4-dihydroquinoxalinones can be conveniently converted into 2-(trifluoromethyl)-1,2,3,4-tetrahydroquinoxalines in excellent yields without loss of optical purity.


 Please wait while we load your content...
Please wait while we load your content...