Recent advances in photocatalytic C–S/P–S bond formation via the generation of sulfur centered radicals and functionalization
Abstract
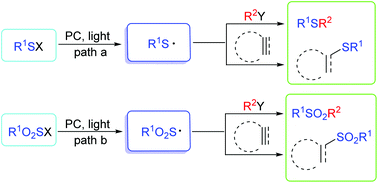
Thioyl and sulfonyl radicals are usually produced from various thiols and sulfonyl derivatives in high efficiency by single-electron-transfer (SET) oxidation. The generated sulfur (thioyl/sulfonyl) radicals are also highly reactive intermediates having various applications in the construction of organosulfur compounds in the field of organic synthesis. Recently, photoredox-catalyzed C–S/P–S bond formation via the generation of sulfur centered radicals has been studied extensively. In the photoredox catalytic process, a variety of S–H, S–S, S–C, S–N, and S–X (F, Cl, Br, I) bonds, and even active sulfone-containing skeletons can be easily transformed into the corresponding thioyl/sulfonyl radicals. Some of these transformations are achieved by a combination of photoredox catalysts (i.e., TiO2, Bi2O3, eosin Y, fac-[Ir(ppy)3], [Ru(bpy)3]2+) and other catalysts such as strong bases, Lewis acids, organocatalysts and transition metal catalysts. Compared with previous methods, photoredox catalysis is inexpensive and features the advantages of high efficiency and easy utilization in addition to being environmentally-benign. In this review, we have focused on the research on photoredox-catalyzed C–S/P–S bond formation via the generation of thioyl/sulfonyl radicals and further functionalization in the past few years. We hope to offer chemists the tools to open the door for further progress in organsulfur chemistry.
- This article is part of the themed collections: FOCUS: Radical-involved chemical transformations and 2019 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...