Chemoselective N-arylation of aminobenzene sulfonamides via copper catalysed Chan–Evans–Lam reactions†
Abstract
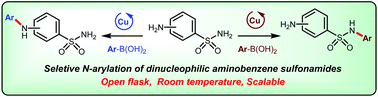
Cu-Catalysed Chan–Evans–Lam cross-coupling reactions between unprotected aminobenzene sulfonamides and arylboron nucleophiles are explored herein. Chemoselective N-arylation of aminobenzene sulfonamides could be simply enabled by the adjustment of reaction variables, such as the sources of aerobic oxidative Cu catalysis, solvents and bases. The arylation processes could be well regulated to occur on either amino or sulfonamide nitrogen atoms at room temperature under open flask conditions.


 Please wait while we load your content...
Please wait while we load your content...