Thermal-mediated catalyst-free heterolytic cleavage of 3-halooxindoles: rapid access to 3-functionalized-2-oxindoles†
Abstract
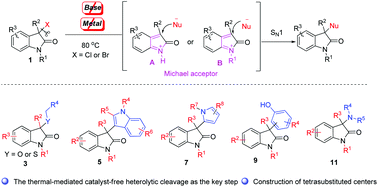
Herein a previously unreported catalyst-free SN1 reaction of the 3-halooxindoles to build 3-functionalized-2-oxindoles is described. The present protocol utilizes the thermal-mediated heterolytic cleavage of the 3-halooxindoles to generate a highly electrophilic 1-H-o-azaxylylene-1-ium A or 1-alkyl-o-azaxylylene-1-ium B intermediate as the Michael acceptor, avoiding the utilization of an additional base and metal as promoters, and providing a diversity-oriented approach to a series of 3-functionalized-2-oxindoles bearing a tetrasubstituted center in high yields (up to 93% yield). This method could provide libraries of structurally diverse and medicinally important small molecules that could aid the search for new bioactive molecules.


 Please wait while we load your content...
Please wait while we load your content...