Novel potential artificial MRSA DNA intercalators: synthesis and biological evaluation of berberine-derived thiazolidinediones†
Abstract
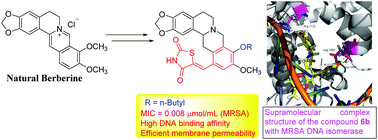
A series of berberine-derived thiazolidinediones as novel potential artificial DNA intercalators were developed via five steps from natural berberine. The biological evaluation showed that some of the target molecules exhibited good inhibitory potencies against the tested bacteria and fungi. Noticeably, compound 6b displayed excellent antibacterial activity with a quite low MIC value of 0.008 μmol mL−1 against MRSA. This compound not only showed low toxicity to hepatocyte LO2 cells and slow development of bacterial resistance, but also displayed efficient membrane penetrability. The DNA binding efficacy was investigated using molecular modeling and UV-vis absorption experiments. The docking study pointed out the specific binding sites and non-covalent interactions of butyl derivative 6b with MRSA DNA isomerase. The active molecule 6b could block DNA replication by intercalating into MRSA DNA to form a supramolecular complex and thus suppress the growth of MRSA strain. Quantum chemical studies were also performed to understand the relationship between biological activity and molecular structure. It was found that the highly active compound 6b could be supramolecularly transported by human serum albumin through hydrophobic interactions and hydrogen bonds. Furthermore, the drug combination of compound 6b with norfloxacin and fluconazole could enhance the antimicrobial efficacy.


 Please wait while we load your content...
Please wait while we load your content...