Iron-catalyzed boration of cinnamyl carbonates: a highly stereoselective approach to cyclopropylboronates†
Abstract
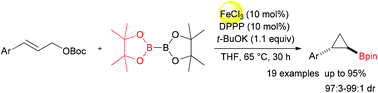
An efficient approach to cyclopropylboronates via iron-catalyzed boration/cyclopropylation of cinnamyl carbonates is developed. The reactions of cinnamyl carbonates and B2pin2 (bis(pinacolato)diboron) proceed smoothly in tetrahydrofuran, catalyzed by ferric chloride/DPPP (1,3-bis(diphenlphosphino)propane). Thus, a wide range of cyclopropylboronates with broad functional-group compatibility was obtained in moderate to high yields (50%–95%) with excellent stereoselectivities (97 : 3–99 : 1 dr). This reaction represents the first iron-catalyzed boration of cinnamyl esters to synthesize cyclopropylboronates.


 Please wait while we load your content...
Please wait while we load your content...