The effect of different environments on excited-state intramolecular proton transfer in 4′-methoxy-3-hydroxyflavone
Abstract
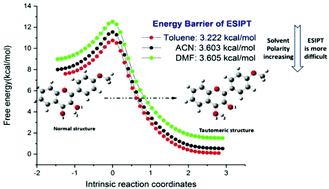
Recently, the fluorescence properties of 4′-methoxy-3-hydroxyflavone dye in different solvents were observed experimentally (A. L. Skilitsi, D. Agathangelou, L. Shulov, J. Conyard, S. Haacke, Y. Mely, A. Klymchenko and J. Leonard, Phys. Chem. Chem. Phys., 2018, 20, 7885–7895). However, corresponding reaction mechanisms were required. In this research, we investigated the effect of different solvent environments on excited-state intramolecular proton transfer for the first time for 4′-methoxy-3-hydroxyflavone. The calculated absorption and emission spectral values matched the experimental data. Hydrogen bond lengths and angles were calculated to confirm the excited state hydrogen bond strengthening mechanism. The frontier molecular orbitals displayed a redistribution of electron density in different solvents, affecting the photophysical properties of this dye. The infrared vibrational spectra and the RDG isosurfaces were analyzed, and illustrated that in the excited state the intensity of the hydrogen bond decreased gradually as the solvent polarity increased. In addition, the potential energy curves indicated that it was increasingly difficult for excited state intramolecular proton transfer reaction to occur in these solvents in the following order: toluene → ACN → DMF.


 Please wait while we load your content...
Please wait while we load your content...