Regiospecific construction of diverse and polyfunctionalized γ-pyrone cores by indium(iii)-catalyzed annulation of diazodicarbonyls with active methylenes, 4-hydroxycoumarins, or 4-hydroxyquinolinone†
Abstract
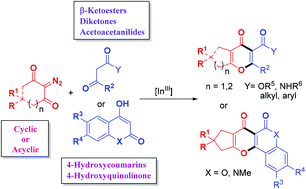
An efficient and novel construction of diverse and polyfunctionalized γ-pyrones has been developed by InBr3-catalyzed annulation of various cyclic and acyclic diazo compounds with multifarious active methylenes, 4-hydroxycoumarins, or 4-hydroxyquinolinone. This technique proceeds through a cascade of carbene generation/ketene formation/conjugate addition/intramolecular cyclization/elimination reactions. In addition, transformation of the synthesized compound for further functionalization is realized.


 Please wait while we load your content...
Please wait while we load your content...