Preparation of pyridyltriazole ruthenium complexes as effective catalysts for the selective alkylation and one-pot C–H hydroxylation of 2-oxindole with alcohols and mechanism exploration†
Abstract
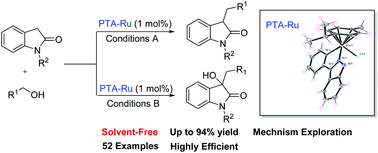
Pyridyltriazole-ligated ruthenium(II) complexes have been designed and synthesized, which were characterized by X-ray crystallography. The resulting complexes were revealed to have good stability through TG experiments and effective catalytic reactivity for selective alkylation and one-pot C–H hydroxylation of 2-oxindole with a variety of primary alcohols, via a hydrogen-borrowing process. The mechanism experiments revealed that the catalytic activity of these catalysts was enhanced by non-coordinating anions. Isotopic labelling experiments were further explored to understand these transformations. In comparison with previous ruthenium systems, these catalysts are not only easily synthesized with cheap triazole as the ligand, but also triazole ruthenium complexes are effective new catalysts for hydrogen-borrowing reaction. This provided an efficient and green method for the synthesis of valuable 3-functionalized-2-oxindoles, 3-functionalized-3-hydroxy-2-oxindoles and quaternary α-hydroxy carbonyl compounds with excellent yields for the first time.


 Please wait while we load your content...
Please wait while we load your content...