Long distance unconjugated agostic-assisted 1,5-H shift in a Ru-mediated Alder-ene type reaction: mechanism and stereoselectivity†
Abstract
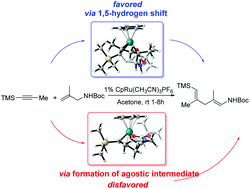
While the mechanisms of transition metal-catalyzed coupling reactions have received extensive attention, the extent to which these apply to catalytic Alder-ene-type reactions remains unclear. A novel 1,5-H shift mechanism for a Ru-catalyzed Alder-ene type alkene–alkyne coupling reaction was examined by density functional theory (DFT). This reaction begins with a cyclometallation between alkene and alkyne to form a ruthenacyclopentene. Then, 1,5-H shift generates an olefin coordination intermediate. Sequential ligand exchanges construct the final product and regenerate the active catalyst. Results show that a pathway through a [3 + 2] cyclometallation and 1,5-H shift step is favored over the traditional cycloaddition – β-hydride elimination and reductive elimination route reported previously. The stereoselectivity of the product is also validated and the results show that it is predominately controlled by the energy differences in both the cyclometallation and the 1,5-H shift step. Regioselectivity is mainly controlled by electronic effect and six-membered ring tension.


 Please wait while we load your content...
Please wait while we load your content...