Synthesis of 6-(sulfonylmethyl)phenanthridines through a reaction of aryldiazonium tetrafluoroborates, sulfur dioxide, and vinyl azides†
Abstract
Synthesis of 6-(sulfonylmethyl)phenanthridines through a three-component reaction of aryldiazonium tetrafluoroborates, a sulfur dioxide surrogate of DABCO·(SO2)2, and vinyl azides under metal- and additive-free conditions is achieved. The arylsulfonyl radicals generated in situ would initiate the sulfonylation of vinyl azides. Subsequent intramolecular cyclization and deprotonation would provide 6-(sulfonylmethyl)phenanthridines in good yields. High efficiency under extremely mild conditions with the insertion of sulfur dioxide through a radical process is observed.
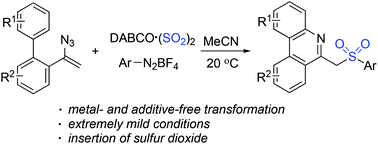


 Please wait while we load your content...
Please wait while we load your content...