Copper-catalyzed radical Heck type cyclization: a three-component reaction of DABCO·(SO2)2, aryldiazonium tetrafluoroborates and dienes toward sulfonated benzo- seven-membered nitrogen heterocycles†
Abstract
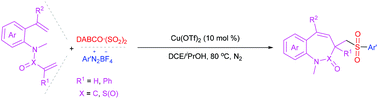
A three-component reaction of the sulfur dioxide surrogate of DABCO·(SO2)2, aryldiazonium tetrafluoroborates and dienes has been developed, affording a series of sulfonated benzo- seven-membered nitrogen heterocycles in moderate to good yields. This procedure was triggered by the insertion of an in situ formed arylsulfonyl radical derived from DABCO·(SO2)2 and aryldiazonium tetrafluoroborates into electron-deficient vinyl, proceeding with a halogen-free radical Heck type reaction during cyclization.


 Please wait while we load your content...
Please wait while we load your content...