Cobalt-catalyzed directed ortho-methylation of arenes with methyl tosylate†
Abstract
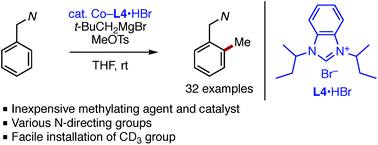
A cobalt-catalyzed directed ortho C–H methylation reaction of arenes has been achieved using readily available methyl tosylate as a methylating agent. An in situ-generated cobalt–N-heterocyclic carbene catalyst in combination with neopentylmagnesium bromide promotes the methylation at room temperature. The reaction is applicable to various substrates bearing nitrogen directing groups such as N-aryl imine, N–H imine, and 2-pyridyl groups. The present protocol also allows for facile introduction of a trideuteriomethyl group into arenes.


 Please wait while we load your content...
Please wait while we load your content...