Expanding the scope of peropyrenes and teropyrenes through a facile InCl3-catalyzed multifold alkyne benzannulation†
Abstract
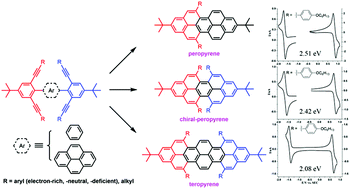
Herein, we report a facile synthesis of bay-region-functionalized peropyrenes and teropyrenes through an InCl3-catalyzed double or quadruple benzannulation reaction of alkynes. This method also allows for the formation of persistently twisted, and thus chiral, peropyrenes. In contrast to using Brønsted acids to facilitate the alkyne benzannulation reaction, these InCl3-catalyzed reactions are capable of cyclizing electron-rich, electron-poor, and even alkyl-substituted alkynes; this drastically improves the scope of products that can be accessed. X-ray crystallographic analysis showed that the substituents in the bay-region play an important role, not only in the crystal packing, but also for their chirality in the solid-state. The excellent solubility and broad scope of both the peropyrenes and teropyrenes obtained by this method, enable us to fully study their interesting optical and electrochemical properties for the first time.


 Please wait while we load your content...
Please wait while we load your content...
