Diastereoselective synthesis of cis-1,3-disubstituted isoindolines via a highly site-selective tandem cyclization reaction†
Abstract
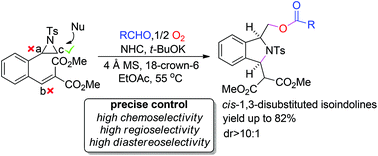
A highly site-selective tandem reaction involving regioselective ring opening of aziridines and Michael addition of electron-deficient alkenes has been described. O-atom nucleophiles, produced by the reaction of aldehydes and NHCs in open air, preferred to attack the less hindered aziridine carbons, followed by further intramolecular aza-Michael addition to afford a series of cis-1,3-disubstituted isoindolines in good yields with good diastereoselectivities. High selectivities, high efficiency and mild conditions made this tandem reaction very suitable for the rapid construction of libraries of isoindoline compounds.


 Please wait while we load your content...
Please wait while we load your content...